One-Hour AI Resistance Prediction Cuts Sepsis Deaths and Drug Costs
The inappropriate use of antibiotics accelerates antimicrobial resistance and fuels the rise of so-called “superbugs,” now regarded as an invisible public-health storm following the COVID-19 pandemic. As superbugs are highly resistant to treatment, and rapid tools for pathogen identification were historically unavailable, physicians often had to rely on experience when prescribing antibiotics. Inaccurate prescribing not only strengthens resistance but can also lead to large-scale infections if mishandled.
China Medical University Hospital (CMUH) has developed an Intelligent Antimicrobial Platform, using artificial intelligence to race against time. Compared with conventional laboratory culture and identification, the platform delivers results 24–72 hours earlier, identifying antimicrobial resistance within one hour. It also sends text-message alerts recommending the most appropriate antibiotics, helping clinicians seize the golden treatment window to save lives.
CMUH Superintendent Der-Yang Cho recalled watching a 2015 TED Talk by Bill Gates, who warned that the greatest future threat to humanity would not be war or missiles, but microorganisms, particularly antibiotic-resistant bacteria. Likewise, the World Health Organization (WHO) has warned that if antimicrobial resistance is not addressed, up to 10 million people could die annually from bacterial infections by 2050, surpassing cancer-related deaths.

Missing the Golden Window: How Antibiotic Misuse Breeds Superbugs
According to Cho, the rampant spread of superbugs is primarily driven by antibiotic misuse, which takes two main forms. One is underestimating disease severity, leading to delayed treatment. The other is overestimating severity, such as immediately prescribing the strongest antibiotics, prompting bacterial mutation and resistance.
Since 2017, antibiotic development has slowed significantly, with only 12 new antibiotics approved, and not all are available in Taiwan. During the COVID-19 pandemic, when it was often unclear whether symptoms were caused by viral or bacterial infections, physicians frequently prescribed antibiotics “just in case,” further exacerbating misuse.
Sepsis, a systemic inflammatory response caused by pathogens, can deteriorate rapidly. Standard laboratory culture, pathogen identification, and reporting typically take three to five days, preventing immediate precision treatment. During this delay, patient conditions can change quickly: each one-hour delay in antibiotic administration increases mortality by 7.6 percent.
“Early diagnosis of sepsis is critical,” Cho said. “Our AI had to be different. First, it must be deployable in real clinical settings, not just remain a research project. Second, it must be easy to use. Third, clinicians must want to use it.” Since its launch, the Intelligent Antimicrobial Platform has accumulated 140,000 clinical uses, with physicians now relying on it daily when prescribing antibiotics.
Four Core Functions Enable Early Diagnosis and Rational Antibiotic Use
The platform was developed through cross-disciplinary collaboration, integrating expertise from infectious diseases, pharmacy, laboratory medicine, medical research, information systems, artificial intelligence, and big data. More than 40 professionals participated in building what the team calls a “total solution.”
The platform’s four core functions, displayed on a unified dashboard, support frontline clinicians throughout the care timeline. When a patient arrives at the hospital, before any tests are performed, the system activates the Personal Microbial and Antimicrobial Profile, integrating data from the past six months, including hospitalizations, specimens, cultured organisms, and antibiotic susceptibility.
Approximately six hours after admission, once blood test results are available, the Sepsis Risk and Mortality Prediction function is triggered, using 10 parameters to estimate the risk of sepsis and death. Around 24 hours later, when preliminary culture results are available, the system combines AI with mass spectrometry analysis to activate Rapid Antimicrobial Resistance Prediction, quickly forecasting whether the pathogen is drug-resistant.
Finally, the Clinical Decision Support System (CDSS) sends text-message alerts to frontline physicians, notifying them of predicted resistant strains, such as carbapenem, resistant Klebsiella pneumoniae (CRKP) and methicillin-resistant Staphylococcus aureus (MRSA). The system will then recommend the most appropriate antibiotics.
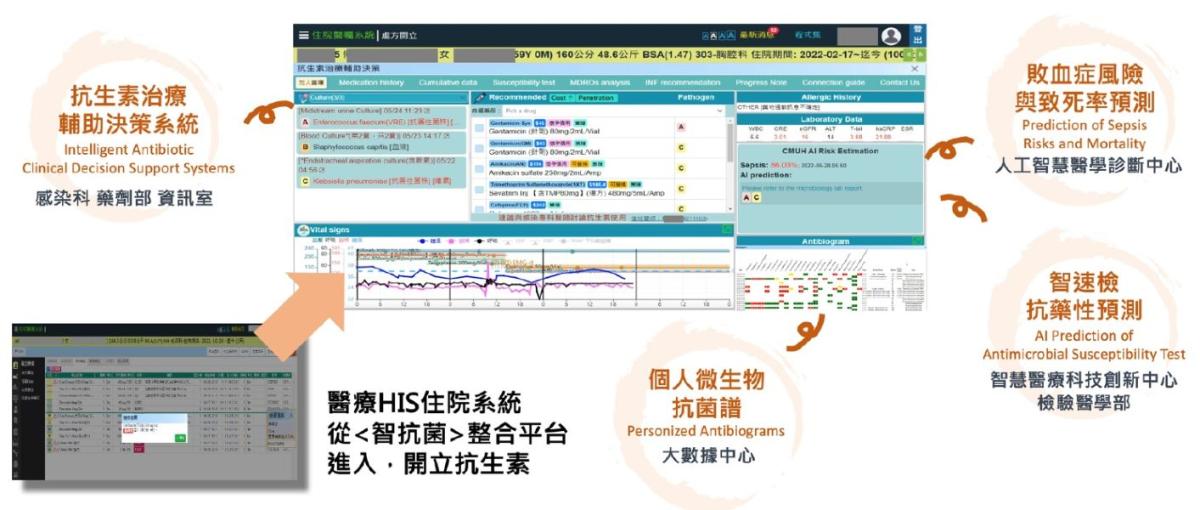
One-Hour Resistance Prediction Powered by AI and Mass Spectrometry
The platform’s core lies in its Rapid Antimicrobial Resistance Prediction function. It incorporates eight high-mortality resistant strains commonly identified by the WHO, Taiwan’s Centers for Disease Control, and CMUH intensive care units, building AI-based mass spectrometry classification models.
According to Jiaxin Yu of the hospital's AI innovation center, bacterial samples are first analyzed by mass spectrometry to generate protein “fingerprints,” accelerating pathogen identification. Differences in protein signals between resistant and non-resistant strains are then used as features to train AI recognition models.
In the past, antibiotic susceptibility testing would require an additional 24–48 hours after pathogen identification. Now, by linking mass spectrometry data with susceptibility information through AI, resistance can be predicted within one hour, allowing a single mass spectrometer to perform the work of both a mass spectrometer and a susceptibility testing system.
“The goal is to give physicians accurate diagnoses early, so they can select the right antibiotics,” said Wei-Cheng Chen of the Respiratory Intensive Care Unit. “Patients often ask for the most expensive or strongest drugs, but the platform’s purpose is to ensure antibiotics are used rationally.”
Reduced Mortality and Antibiotic Costs with 85% Prediction Accuracy
Since integration into the hospital information system (HIS) in February 2022, the platform’s four functions have been rolled out in phases and embedded into clinical workflows. It has helped reduce sepsis mortality by 12 percent and has generated 18 published papers.
Overall resistance prediction accuracy averages 85 percent, while MRSA prediction accuracy reaches 95 percent, benefiting from large datasets of more than 10,000 strains.
Cho also noted that while antibiotics typically account for about 30 percent of total drug costs in regional hospitals, CMUH has reduced this figure to around 20 percent, thanks to earlier and more precise prescribing.

From Internal Adoption to Cross-Hospital Validation and Commercialization
Beyond hospital-wide deployment, CMUH has provided the platform free of charge to four partner hospitals, namely National Taiwan University Hospital Yunlin Branch, Tungs’ Taichung MetroHarbor Hospital (Shalu Campus), Chi Mei Medical Center, and Fengyuan Hospital, to conduct cross-institutional validation.
Through federated learning, multiple institutions collaboratively train machine-learning models without sharing raw data, protecting privacy, reducing transmission costs, and accelerating learning.
Identifying unmet clinical needs requires intensive early collaboration with clinicians, Yu said. “Once the system is live, frontline feedback allows us to rapidly iterate and improve the models.”
Editor’s Note: This article features the Bronze Award recipient of the 25th National Biotechnology and Medicine Care Quality Award (2022), Healthcare Institution Category, Smart Healthcare Group. All titles and positions mentioned reflect the interviewees’ roles at the time of the interviews.






